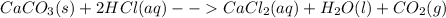The reaction between calcium carbonate (caco3) and hcl produces calcium chloride (cacl2), carbon dioxide (co2), and water (h2o).what happens when the concentration of hydrogen chloride (hcl) molecules is doubled in this reaction? caco3 + 2hcl → cacl2 + co2 + h2o when the hydrogen chloride concentration doubles, the number of collisions between the reactants (increases, decreases, remains constant) , which causes the rate of the forward reaction to (increase, decrease, remain constant)

Answers: 1
Similar questions

Chemistry, 12.07.2019 00:40, AM28
Answers: 1

Chemistry, 02.09.2019 16:20, eddrekas8564
Answers: 2

Chemistry, 09.10.2019 03:10, MGA20078
Answers: 1

Chemistry, 23.10.2019 18:00, jesseemartinez22
Answers: 1
Do you know the correct answer?
The reaction between calcium carbonate (caco3) and hcl produces calcium chloride (cacl2), carbon dio...
Questions in other subjects:

Mathematics, 09.02.2021 21:40

Mathematics, 09.02.2021 21:40


Mathematics, 09.02.2021 21:40

History, 09.02.2021 21:40

Mathematics, 09.02.2021 21:40




Mathematics, 09.02.2021 21:40