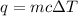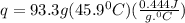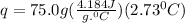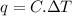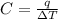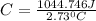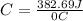Chemistry, 03.07.2019 13:00, xmanavongrove55
Acalorimeter contained 75.0 g of water at 16.95 c. a 93.3-g sample of iron at 65.58 c was placed in it, giving a final temperature of 19.68 c for the system. calculate the heat capacity of the calorimeter. specific heats are 4.184 j/g c for h2o and 0.444 j/g c for fe.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 23.06.2019 03:30, HalpMahOnMahH0meW0rk
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
Do you know the correct answer?
Acalorimeter contained 75.0 g of water at 16.95 c. a 93.3-g sample of iron at 65.58 c was placed in...
Questions in other subjects:



Geography, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00


Chemistry, 18.03.2021 03:00



Health, 18.03.2021 03:00


 .
. for iron metal = 65.58 - 19.68 = 45.9 degree C
for iron metal = 65.58 - 19.68 = 45.9 degree C