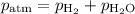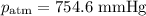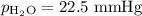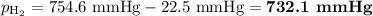Chemistry, 03.07.2019 13:30, nicky123415
Mass of metal=0.0291. volume of gas collected over water=25.67ml. temperature=24.1c. atmospheric pressure=754.6mmhg. note: metal and gas have a 1: 1 ratio in the balanced equation. the formula weight of the metal is? steps. 1) calculate partial pressure of hydrogen. 2) calculate moles of hydrogen. 3) calculate moles of metal. 4) calculate atomic mass of metal. 5) calculate relative average deviation in ppt

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, dijonmckenzie3
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3


Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
Do you know the correct answer?
Mass of metal=0.0291. volume of gas collected over water=25.67ml. temperature=24.1c. atmospheric pre...
Questions in other subjects:

Social Studies, 28.06.2019 08:30

English, 28.06.2019 08:30




Social Studies, 28.06.2019 08:30

Biology, 28.06.2019 08:30


English, 28.06.2019 08:30