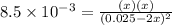Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 23:30, ninilizovtskt
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
Do you know the correct answer?
For the equilibrium 2 ibr (g) i2 (g) + br2 (g) kp=8.5 ×10-3 at 150 oc. if 0.025 atm of ibr is place...
Questions in other subjects:

Social Studies, 26.06.2019 00:30



Mathematics, 26.06.2019 00:30




History, 26.06.2019 00:30

Mathematics, 26.06.2019 00:30


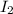 and
and 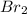 is 0.0211 atm, 0.00195 atm and 0.00195 atm respectively.
is 0.0211 atm, 0.00195 atm and 0.00195 atm respectively.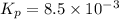
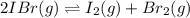
![K_p=\frac{[P_I_2][P_{Br}_2]}{[P_{IBr}]^2}](/tpl/images/0046/9546/983fc.png)