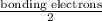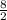Chemistry, 03.07.2019 16:30, EssenceBlocker144
Consider the lewis structure for the polyatomic oxyanion shown here, where x is an element from the third period (na−ar). by changing the overall charge, n, from 1- to 2- to 3- we get three different polyatomic ions. element x is surrounded by 4 oxygen atoms bonded to it with full octets. a)for each of these ions identify the central atom, x. arrange your answers in order increasing n. b)for each of these ions determine the formal charge of the central atom, x. arrange your answers in order increasing n. i really have no idea how to solve this. any would be greatly appreciated!

Answers: 1
Similar questions

Chemistry, 22.06.2019 15:30, sanchez7489
Answers: 1

Chemistry, 28.08.2019 04:30, RayshawnBoulware
Answers: 1
Do you know the correct answer?
Consider the lewis structure for the polyatomic oxyanion shown here, where x is an element from the...
Questions in other subjects:

English, 06.05.2020 21:11









Mathematics, 06.05.2020 21:11