

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, ineedhelp773
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases. as altitude increases, air density increases. air pressure and density are lowest at sea level. denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 21.06.2019 20:10, cefindley14
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 00:40, btcastongia
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3
Do you know the correct answer?
Adding naoh to an aqueous solution containing ni2+ results in the precipitation ofni(oh)2. the stand...
Questions in other subjects:





Chemistry, 16.10.2019 23:30






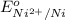 = +0.25 V
= +0.25 V![K_{sp}=[Ni^{2+}][OH^-]^2=1.5\times 10^{-16}](/tpl/images/0048/3070/43bf5.png)
![pH=-log[H^+]=14](/tpl/images/0048/3070/4f928.png)
![[H^+]=10^{-14}](/tpl/images/0048/3070/eec66.png)
![K_w=[H^+][OH^-]=10^{-14}](/tpl/images/0048/3070/f3553.png)
![[OH^-]=\frac{K_w}{[H^+]}=\frac{10^{-14}}{10^{-14}}=1](/tpl/images/0048/3070/8d077.png)
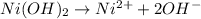
![K_{sp}=[Ni^{2+}][OH^-]^2](/tpl/images/0048/3070/c86a1.png)
![[Ni^{2+}]](/tpl/images/0048/3070/2f342.png) ion.
ion.^2](/tpl/images/0048/3070/f235c.png)
![[Ni^{2+}]=1.5\times 10^{-16}](/tpl/images/0048/3070/989b5.png)
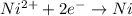
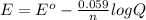
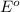 = standard electrode potential of the cell
= standard electrode potential of the cell![E=E^o_{Ni^{2+}/Ni}-\frac{0.059}{n}log\frac{1}{[Ni^{2+}]}](/tpl/images/0048/3070/64772.png)
![E=+0.25V-\frac{0.059}{2}log\frac{1}{[1.5\times 10^{-16}]}](/tpl/images/0048/3070/b7e57.png)





