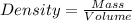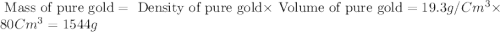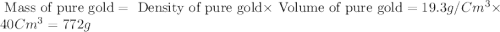Esteban finds that a small bar of pure gold, with a density of 19.3 g/cm³, displaces 80 cm³ of water. he calculates that it's mass is 1,544 g. he then calculates that a smalller bar, with half the volume of the first bar, has a mass half a great. is he correct? explain your reasoning.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
Do you know the correct answer?
Esteban finds that a small bar of pure gold, with a density of 19.3 g/cm³, displaces 80 cm³ of water...
Questions in other subjects:


Computers and Technology, 27.05.2021 06:50








Physics, 27.05.2021 06:50


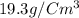
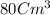 of water that means the Volume of pure gold is equal to the volume of water displaces.
of water that means the Volume of pure gold is equal to the volume of water displaces.