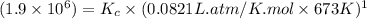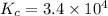Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, kathleendthomas
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
Do you know the correct answer?
If the reaction below has a kp =1.9 × 106 at 400°c, what is kc? n2o(g) + no2(g) 3no(g) a. 2.9 × 10...
Questions in other subjects:



Mathematics, 02.12.2020 04:10

Mathematics, 02.12.2020 04:10

Mathematics, 02.12.2020 04:10


Mathematics, 02.12.2020 04:10


Mathematics, 02.12.2020 04:10

Computers and Technology, 02.12.2020 04:10


 is
is 
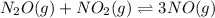
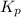 and
and 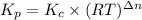

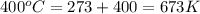
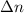 = change in the number of moles of gas = [(3) - (1 + 1)] = 1 (from the chemical reaction)
= change in the number of moles of gas = [(3) - (1 + 1)] = 1 (from the chemical reaction)