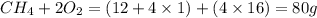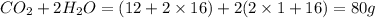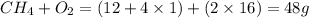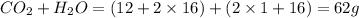Chemistry, 04.07.2019 14:00, ashleyheink3796
When methane, ch4, is combusted, it produces carbon dioxide, co2, according to the unbalanced equation: ch4 + o2 → co2 + h2o. write the balanced equation for this reaction, and explain how it is possible for 10 grams of methane fuel to burn and emit 27 grams of carbon dioxide. discuss whether or not this reaction obeys the law of conservation of mass.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, KennyOaks6230
Which of the following units is not an official si unit
Answers: 1

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Do you know the correct answer?
When methane, ch4, is combusted, it produces carbon dioxide, co2, according to the unbalanced equati...
Questions in other subjects:


Mathematics, 01.04.2021 03:20



Geography, 01.04.2021 03:20


Mathematics, 01.04.2021 03:20

Mathematics, 01.04.2021 03:20

Mathematics, 01.04.2021 03:20

History, 01.04.2021 03:20


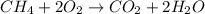
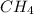 = 16 g/mole
= 16 g/mole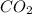 = 44 g/mole
= 44 g/mole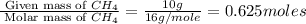
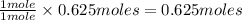 of
of