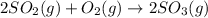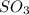How would adding the catalyst nitrogen monoxide (no) affect this reaction? 2so2(g) + o2(g) → 2so3(g) a. no increases the rate at which so3 molecules are formed. b. no reacts with so3 to produce more so2 molecules. c. no decreases collisions between the so2 and o2 molecules. d. no increases the concentration of the so2 and o2 molecules. e. no increases the activation energy of the so2 and o2 molecules.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1


Chemistry, 23.06.2019 01:30, koggebless
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
Do you know the correct answer?
How would adding the catalyst nitrogen monoxide (no) affect this reaction? 2so2(g) + o2(g) → 2so3(g...
Questions in other subjects:






Mathematics, 31.03.2020 20:34


Chemistry, 31.03.2020 20:34