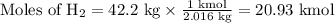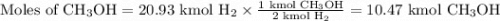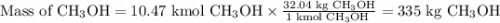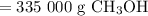Chemistry, 04.07.2019 22:00, addisynshepherd
How much methanol (ch3oh, in grams) can be formed from 42.2 kg of hydrogen? assume excess co. co(g) + 2h2(g) → ch3oh(g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 03:00, amberskids2
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
Do you know the correct answer?
How much methanol (ch3oh, in grams) can be formed from 42.2 kg of hydrogen? assume excess co. co(g)...
Questions in other subjects:

Biology, 08.10.2021 14:00




Mathematics, 08.10.2021 14:00

History, 08.10.2021 14:00





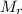 : 2.016 32.04
: 2.016 32.04