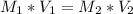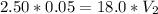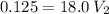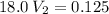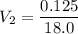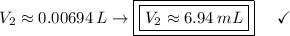In a school’s laboratory, students require 50.0 ml of 2.50 m h2so4 for an experiment, but the only available stock solution of the acid has a concentration of 18.0 m. what volume of the stock solution would they use to make the required solution? use mc018-1.jpg.

Answers: 2
Similar questions

Chemistry, 17.07.2019 04:00, 0gNanaa
Answers: 2

Chemistry, 21.07.2019 18:00, harveyangel123p2tjae
Answers: 2
Do you know the correct answer?
In a school’s laboratory, students require 50.0 ml of 2.50 m h2so4 for an experiment, but the only a...
Questions in other subjects:




History, 11.10.2019 02:00



Mathematics, 11.10.2019 02:00

History, 11.10.2019 02:00