
1.which of the following is not true of polar covalent bonds? a. polar covalent bonds create two poles across the bond. b. polar covalent bonds result from the unequal sharing of electrons. c. electronegativity differences for atoms with polar covalent bonds range between 0.5 and 2.0. d. polar covalent bonds exist between atoms of the same element. 2. which of the following would not increase the rate of most reactions? a. removing an inhibitor b. lowering the concentration of the reactants c. increasing the temperature d. adding a catalyst

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 23:00, genyjoannerubiera
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 00:30, coralaguilar1702
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
Do you know the correct answer?
1.which of the following is not true of polar covalent bonds? a. polar covalent bonds create two p...
Questions in other subjects:

Mathematics, 14.11.2020 01:20



Mathematics, 14.11.2020 01:20


History, 14.11.2020 01:20

Mathematics, 14.11.2020 01:20

English, 14.11.2020 01:20

History, 14.11.2020 01:20

History, 14.11.2020 01:20


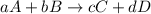
![rate=k[A]^a[B]^b](/tpl/images/0052/8463/e8923.png)




