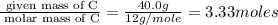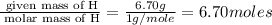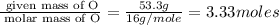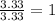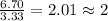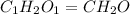Chemistry, 05.07.2019 08:30, hooplikenari
Acompound is 40.0% c, 6.70% h, and 53.3% o by mass. assume that we have a 100.-g sample of this compound. part a what are the subscripts in the empirical formula of this compound? enter the subscripts for c, h, and o, respectively, separated by commas (e. g., 5,6,7).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1


Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
Do you know the correct answer?
Acompound is 40.0% c, 6.70% h, and 53.3% o by mass. assume that we have a 100.-g sample of this comp...
Questions in other subjects:


Mathematics, 18.12.2020 01:00

Arts, 18.12.2020 01:00



Mathematics, 18.12.2020 01:00




History, 18.12.2020 01:00