
Chemistry, 05.07.2019 11:00, Ashley606hernandez
An 8.65 g sample of an unknown group 2a metal hydroxide is dissolved in 85.0 ml of water and titrated with 2.50 m hcl(aq). if it takes 56.9 ml of the acid to reach the end point of the titration (a) what is the molar mass of the metal hydroxide? (b) which of the following is in the metal hydroxide: ca2+, sr2+, ba2+?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, badgirl2005
This is a mixture that has the same composition throughout.
Answers: 1


Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
Do you know the correct answer?
An 8.65 g sample of an unknown group 2a metal hydroxide is dissolved in 85.0 ml of water and titrate...
Questions in other subjects:





Mathematics, 29.08.2020 01:01


History, 29.08.2020 01:01


Mathematics, 29.08.2020 01:01


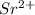 .
.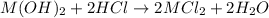
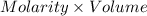
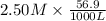
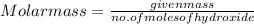
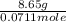
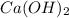 = (40.078 + 34) g/mol = 74.093 g/mol
= (40.078 + 34) g/mol = 74.093 g/mol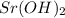 = (87.66 + 34) g/mol = 121.66 g/mol
= (87.66 + 34) g/mol = 121.66 g/mol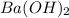 = (137.32 + 34) g/mol = 171.32 g/mol
= (137.32 + 34) g/mol = 171.32 g/mol




