

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:00, catdog5225
What is formed when amino acids form long chains or polymerize
Answers: 1
Do you know the correct answer?
In standardizing the solution of aqueous sodium hydroxide, a chemist overshoots the end point and ad...
Questions in other subjects:






Mathematics, 14.12.2020 22:00


Social Studies, 14.12.2020 22:00


Social Studies, 14.12.2020 22:00


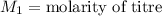 ,
, 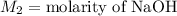
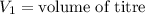 ,
, 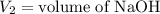
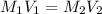
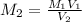 ....(1)
....(1)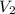 .
.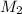 in equation (1), will get lowered , which means that the concentration of NaOH was lower than that of the actual value. Hence underestimated concentration of NaOH.
in equation (1), will get lowered , which means that the concentration of NaOH was lower than that of the actual value. Hence underestimated concentration of NaOH. 



