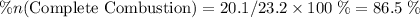Chemistry, 05.07.2019 18:00, amortalstardev
Octane (c8h18) is a component of gasoline. complete combustion of octane yields h2o and co2. incomplete combustion produces h2o and co, which not only reduces the efficiency of the engine using the fuel but is also toxic. in a certain test run, 1.000 gallon (gal) of octane is burned in an engine. the total mass of co, co2, and h2o produced is 11.53 kg. calculate the efficiency of the process; that is, calculate the fraction of octane converted to co2. the density of octane is 2.650 kg/gal.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 00:30, vane6176
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
Do you know the correct answer?
Octane (c8h18) is a component of gasoline. complete combustion of octane yields h2o and co2. incompl...
Questions in other subjects:

Mathematics, 17.09.2019 09:30




Social Studies, 17.09.2019 09:30

History, 17.09.2019 09:30

Spanish, 17.09.2019 09:30

English, 17.09.2019 09:30


Mathematics, 17.09.2019 09:30


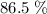 of octane had been converted to carbon dioxide CO₂.
of octane had been converted to carbon dioxide CO₂.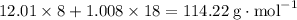
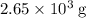 , which corresponds to
, which corresponds to 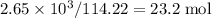 of octane.
of octane.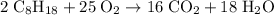
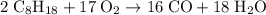
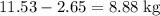 heavier than that of the octane supplied. Thus
heavier than that of the octane supplied. Thus 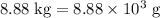 of oxygen were consumed in the combustion. There are
of oxygen were consumed in the combustion. There are 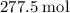 of oxygen molecules in
of oxygen molecules in 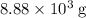 of oxygen.
of oxygen. (
(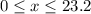 ). The number of moles of octane that had undergone incomplete combustion through the second equation would thus equal
). The number of moles of octane that had undergone incomplete combustion through the second equation would thus equal 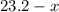 .
.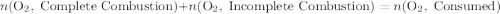
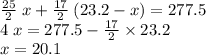
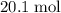 out of the 23.2 moles of octane had undergone complete combustion to produce carbon dioxide.
out of the 23.2 moles of octane had undergone complete combustion to produce carbon dioxide.