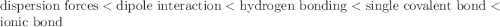Chemistry, 05.07.2019 18:30, jaylan11brown
Place the following types of attraction weakest to strongest: dispersion ionic bond single covalent bond hydrogen bond dipole interaction

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 16:00, ayahabdulhaqq2
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1
Do you know the correct answer?
Place the following types of attraction weakest to strongest: dispersion ionic bond single covalen...
Questions in other subjects:







History, 20.07.2019 15:30

Social Studies, 20.07.2019 15:30

Biology, 20.07.2019 15:30