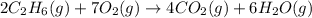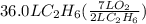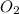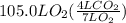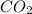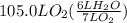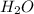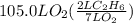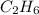Chemistry, 05.07.2019 22:00, aidendespatieshakim
How many liters of gas will be in the closed reaction flask when 36.0l of ethane (c2h6) is allowed to react with 105.0l of oxygen (under constant pressure and temperature) to form carbon dioxide gas and water vapor? assume ideal gas behavior.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
Do you know the correct answer?
How many liters of gas will be in the closed reaction flask when 36.0l of ethane (c2h6) is allowed t...
Questions in other subjects:

Mathematics, 03.03.2021 22:40

Mathematics, 03.03.2021 22:40


Mathematics, 03.03.2021 22:40



Mathematics, 03.03.2021 22:40