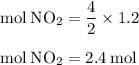Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, hannahkelly3618
How many moles are equivalent to 55.5g of nano3
Answers: 1

Chemistry, 21.06.2019 22:40, wiltseliz4800
What does the process of natural selection involve
Answers: 1


Chemistry, 22.06.2019 11:00, Usman458
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1
Do you know the correct answer?
For the reaction shown, calculate how many moles of no2 form when each amount of reactant completely...
Questions in other subjects:


Mathematics, 19.02.2021 22:10

Geography, 19.02.2021 22:10


Mathematics, 19.02.2021 22:10


French, 19.02.2021 22:10

Mathematics, 19.02.2021 22:10


Mathematics, 19.02.2021 22:10