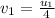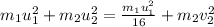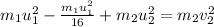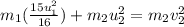Chemistry, 06.07.2019 05:00, daydallas01
The collision between two gas molecules with different masses results in the velocity of the first molecule decreasing by 1/4. if the collision is elastic, which of the following statements is true? a. the velocity of the second molecule will increase by a factor of 4. b. the velocity of the second molecule will increase by a factor of 16. c. the velocity of the second molecule will decrease by a factor of 16. d. the velocity change of the second molecule depends on both the mass and the velocity of the first molecule. explain why it is the right answer too

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1


Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
Do you know the correct answer?
The collision between two gas molecules with different masses results in the velocity of the first m...
Questions in other subjects:

Mathematics, 03.02.2021 03:20




Mathematics, 03.02.2021 03:20

Arts, 03.02.2021 03:20


Social Studies, 03.02.2021 03:20


Spanish, 03.02.2021 03:20


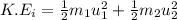
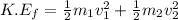
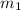 = mass of first molecule
= mass of first molecule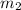 = mass of second molecule
= mass of second molecule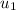 = initial velocity of first molecule
= initial velocity of first molecule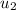 = initial velocity of second molecule
= initial velocity of second molecule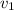 = final velocity of first molecule
= final velocity of first molecule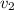 = final velocity of second molecule
= final velocity of second molecule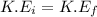
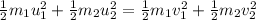
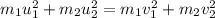 .....(1)
.....(1)