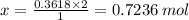Chemistry, 06.07.2019 06:30, jscout2468
You are given the balanced chemical equation: c4h4 + 5o2 4co2 + 2h2o. if 0.3618 moles of c4h4 are allowed to react with 1.818 moles of o2, and this is the only reaction which occurs, what is the maximum mass of water that could be produced?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3


Chemistry, 22.06.2019 12:00, sophiaa23
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
Do you know the correct answer?
You are given the balanced chemical equation: c4h4 + 5o2 4co2 + 2h2o. if 0.3618 moles of c4h4 are a...
Questions in other subjects:



Mathematics, 05.06.2021 07:10



Mathematics, 05.06.2021 07:10

Chemistry, 05.06.2021 07:10


Mathematics, 05.06.2021 07:10

Mathematics, 05.06.2021 07:10