
At 2000 ∘c the equilibrium constant for the reaction 2no(g)⇌n2(g)+o2(g) is kc=2.4×103. you may want to reference (pages 641 - 644) section 15.6 while completing this problem. part a if the initial concentration of no is 0.175 m, what is the equilibrium concentration of no? g

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, CauseWhyNot6235
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1


Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
Do you know the correct answer?
At 2000 ∘c the equilibrium constant for the reaction 2no(g)⇌n2(g)+o2(g) is kc=2.4×103. you may want...
Questions in other subjects:

Mathematics, 12.03.2021 18:50


History, 12.03.2021 18:50

Biology, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50


Mathematics, 12.03.2021 18:50




 .
.
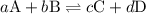
![{K_{\text{c}}}=\dfrac{{{{\left[ {\text{C}} \right]}^c}{{\left[ {\text{D}} \right]}^d}}}{{{{\left[ {\text{A}} \right]}^a}{{\left[ {\text{B}} \right]}^b}}}](/tpl/images/0058/9264/8d53d.png)
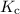 is the equilibrium constant.
is the equilibrium constant.
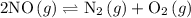
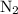 and
and 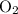 become x at equilibrium.
become x at equilibrium.
![{K_{\text{c}}}=\dfrac{{\left[ {{{\text{N}}_2}} \right]\left[{{{\text{O}}_2}} \right]}}{{{{\left[ {{\text{NO}}} \right]}^2}}}](/tpl/images/0058/9264/f4ed4.png) …… (1)
…… (1)  for
for 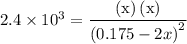 …… (2)
…… (2)

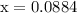
![\begin{aligned}\left[ {{\text{NO}}} \right]&= 0.175 - 2\left( {0.0866} \right)\\&= {\text{ 0}}{\text{.0018 M}}\\\end{aligned}](/tpl/images/0058/9264/23268.png)





