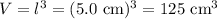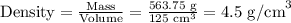Chemistry, 06.07.2019 23:30, s3r3naagarc1a
Asquare block made of a very strong metal has a mass of 0.56375 kilograms. the length of each side block is 5 .0 cm. a) caculate the volume of the block in cm3. b) calculate the density of the block ing /ml.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Do you know the correct answer?
Asquare block made of a very strong metal has a mass of 0.56375 kilograms. the length of each side b...
Questions in other subjects:

Mathematics, 11.05.2021 05:20

Mathematics, 11.05.2021 05:20

Biology, 11.05.2021 05:20

Mathematics, 11.05.2021 05:20


Mathematics, 11.05.2021 05:20



History, 11.05.2021 05:20