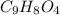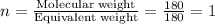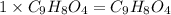Chemistry, 07.07.2019 02:30, jesh0975556
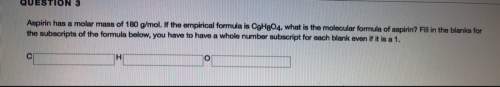
Aspirin has a molar mass of 180g/mol. if the empirical formula is c9h8o4, what is the molecular formula of aspirin ? fill in the blanks for the subscripts of the formula below. you have to have a whole number subscript for each blank even if it is a 1. c__h__o__

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1
Do you know the correct answer?
Aspirin has a molar mass of 180g/mol. if the empirical formula is c9h8o4, what is the molecular form...
Questions in other subjects:



Arts, 10.11.2020 21:30


Mathematics, 10.11.2020 21:30

Arts, 10.11.2020 21:30

Physics, 10.11.2020 21:30

Physics, 10.11.2020 21:30

Mathematics, 10.11.2020 21:30