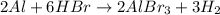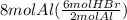Chemistry, 07.07.2019 03:00, josebienka
Consider the reaction 2al + 6hbr → 2albr3 + 3h2. if 8 moles of al react with 8 moles of hbr, what is the limiting reactant? al albr3 h3 hbr

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1


Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
Do you know the correct answer?
Consider the reaction 2al + 6hbr → 2albr3 + 3h2. if 8 moles of al react with 8 moles of hbr, what is...
Questions in other subjects: