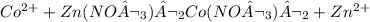Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, jbarbie3
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 23.06.2019 01:00, liamgreene90
The time that is taken by neptune once around the sun is called
Answers: 1
Do you know the correct answer?
Which of the following will produce a precipitate? a) rh3+(aq) + k3po4(aq) b) nb3+(aq) + li2co3 (a...
Questions in other subjects:

Health, 22.11.2019 11:31

Mathematics, 22.11.2019 11:31



Mathematics, 22.11.2019 11:31

Mathematics, 22.11.2019 11:31



Mathematics, 22.11.2019 11:31