Chemistry, 07.07.2019 04:30, IsabellaGracie
What would be the final temperature of the system if 21.2 g of lead at 113 ◦c is dropped into 14.8 g of water at 28.9 ◦c in an insulated container? the specific heat of lead is 0.128 j/g◦c.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 02:30, ijustneedhelp29
Which of the following statements are incorrect?
Answers: 3
Do you know the correct answer?
What would be the final temperature of the system if 21.2 g of lead at 113 ◦c is dropped into 14.8 g...
Questions in other subjects:





Mathematics, 16.04.2020 03:24


History, 16.04.2020 03:24



History, 16.04.2020 03:25


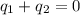
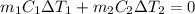
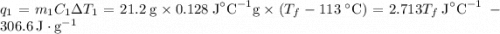
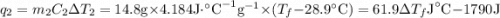
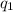 and
and 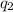 and combinibg like terms, we get
and combinibg like terms, we get![168.0\text{T}_{f}\: ^{\circ}\text{C} ^{-1}\:- 2096 = 0]\\](/tpl/images/0060/4306/42ee8.png)