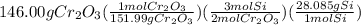Chemistry, 07.07.2019 13:30, lazavionadams81
The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3si(> 4cr(l) + 3sio2 (s) the reaction is begun with 167.00 g of si and 146.00 g of cr2o3. how many grams of the excess reactant are left after the reaction is complete? i found which one was the l. r but i can't figure out how to find the excess amount of the e. r. will be greatly

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3


Do you know the correct answer?
The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3...
Questions in other subjects:

Mathematics, 29.01.2021 04:20

Mathematics, 29.01.2021 04:20





Mathematics, 29.01.2021 04:20

Mathematics, 29.01.2021 04:20

Advanced Placement (AP), 29.01.2021 04:20


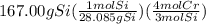
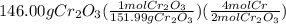
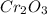 gives the less moles of the product and so it is limiting reactant and hence the excess reactant is Si.
gives the less moles of the product and so it is limiting reactant and hence the excess reactant is Si.