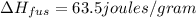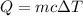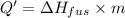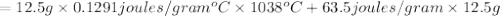Agoldsmith melts 12.4 grams of gold to make a ring. the temperature of the gold rises from 26°c to 1064°c, and then the gold melts completely. if gold’s specific heat is 0.1291 joules/gram degree celsius and its heat of fusion is 63.5 joules/gram, how much energy is gained by the gold? the gold gains a total of joules of energy. i was given this by somebody. no idea what to do with this. qj=(12.4g*(1064-26)°c*0.1291j/g/°c) +(12.4g*63.5j)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
Do you know the correct answer?
Agoldsmith melts 12.4 grams of gold to make a ring. the temperature of the gold rises from 26°c to 1...
Questions in other subjects:





Geography, 21.10.2019 16:20


Business, 21.10.2019 16:20


English, 21.10.2019 16:20

Social Studies, 21.10.2019 16:20