

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, ayahabdulhaqq2
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 21.06.2019 19:00, cutebab4786
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3
Do you know the correct answer?
The compound copper(ii) nitrate is a strong electrolyte. write the reaction when solid copper(ii) ni...
Questions in other subjects:





Mathematics, 05.05.2020 07:34

English, 05.05.2020 07:34




Chemistry, 05.05.2020 07:34


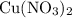 contains copper (II) ions
contains copper (II) ions 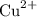 and nitrate
and nitrate 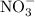 ions.
ions. 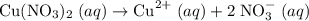
 .
.



