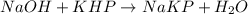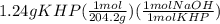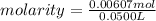Chemistry, 07.07.2019 21:00, nickname9154
If 50.0 ml of naoh solution is required to react completely with 1.24 g khp, what are the molarity and normality of the naoh solution?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
Do you know the correct answer?
If 50.0 ml of naoh solution is required to react completely with 1.24 g khp, what are the molarity a...
Questions in other subjects:

Mathematics, 29.03.2021 07:10

English, 29.03.2021 07:10

Mathematics, 29.03.2021 07:10

Health, 29.03.2021 07:10






World Languages, 29.03.2021 07:10