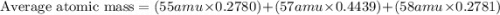Chemistry, 07.07.2019 23:00, a897coleman
An undiscovered element has three naturally occurring isotopes of x-55, x-57, and x-58. isotope x-55 has an abundance of 27.80 % and isotope x-57 has an abundance of 44.39 %. what is the average mass of this element in amu?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2


Chemistry, 23.06.2019 00:30, clairebear66
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 01:30, Nakiahalogn4
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
Do you know the correct answer?
An undiscovered element has three naturally occurring isotopes of x-55, x-57, and x-58. isotope x-55...
Questions in other subjects:


Social Studies, 04.03.2021 22:30



English, 04.03.2021 22:30

English, 04.03.2021 22:30


Mathematics, 04.03.2021 22:30



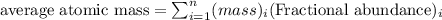 .....(1)
.....(1)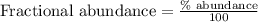
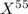 ,
,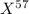 ,
,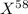 ,
,