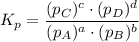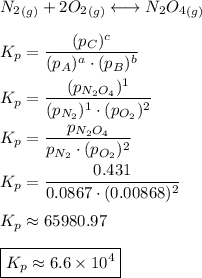Answers: 1
Similar questions




Chemistry, 24.08.2019 00:10, mdbar321
Answers: 1
Do you know the correct answer?
Consider the equilibrium, n2(g) + 2o2(g) < > n2o4(g). calculate the equilibrium constant, kpi...
Questions in other subjects:

English, 18.10.2019 01:30

Mathematics, 18.10.2019 01:30


Spanish, 18.10.2019 01:30



Biology, 18.10.2019 01:30



History, 18.10.2019 01:30