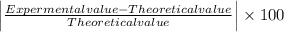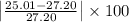Milhouse massed a copper cylinder multiple times. his data is listed below. the "correct" mass of the cylinder had been previously determined to be 27.20 grams. desrcibe the accuracy and precision of the milhouses measurements. weighing 1 - 25.01weighing 2 - 25.22weighing 3 - 25.23weighing 4 - 25.19(part 2) refer to the data above. what was the percent error on milhouses first weighing? percent error = l experimental value - accepted value l / accepted value x 100%

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 00:40, joe7977
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
Do you know the correct answer?
Milhouse massed a copper cylinder multiple times. his data is listed below. the "correct" mass of th...
Questions in other subjects:






History, 24.09.2020 14:01

Biology, 24.09.2020 14:01

Mathematics, 24.09.2020 14:01

Spanish, 24.09.2020 14:01

Mathematics, 24.09.2020 14:01