Chemistry, 08.07.2019 11:00, Brainly264
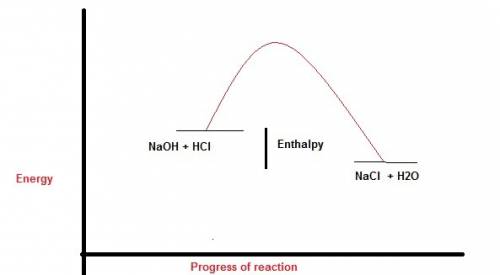
Perhatikan persamaan termokimia berikut. hcl (aq) + naoh (aq) -> nacl (aq) + h2o (l). delta h=-54 kj a. gambarlah diagram tingkat energi untuk reaksi tersebut b. berapakah perubahan entalpi jika 100 ml naoh 1 m ? c. berapakah perubahan entalpi jika 10 ml hcl 1 m direaksikan dengan 20 ml naoh 1 m ?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2


Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
Do you know the correct answer?
Perhatikan persamaan termokimia berikut. hcl (aq) + naoh (aq) -> nacl (aq) + h2o (l). delta h=-5...
Questions in other subjects:




Physics, 22.06.2019 19:30


Mathematics, 22.06.2019 19:30


English, 22.06.2019 19:30