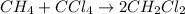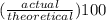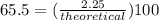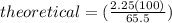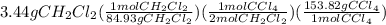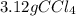Chemistry, 08.07.2019 18:00, bionicboy03120440
It is desired to produce 2.25 grams of dichloromethane (ch2cl2) by the following reaction. if the percent yield of dichloromethane (ch2cl2) is 65.5 %, how many grams of carbon tetrachloride would need to be reacted?carbon tetrachloride methane (ch4)(g) + carbon tetrachloride(g) dichloromethane (ch2cl2)(g)

Answers: 1
Similar questions

Chemistry, 27.07.2019 18:00, alondrasanchezvillan
Answers: 1

Chemistry, 29.07.2019 20:40, aons
Answers: 1

Do you know the correct answer?
It is desired to produce 2.25 grams of dichloromethane (ch2cl2) by the following reaction. if the pe...
Questions in other subjects:

Mathematics, 31.12.2019 20:31

Mathematics, 31.12.2019 20:31

English, 31.12.2019 20:31



Mathematics, 31.12.2019 20:31

History, 31.12.2019 20:31


Mathematics, 31.12.2019 20:31

Biology, 31.12.2019 20:31