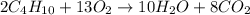Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Do you know the correct answer?
Disposable lighters contain liquid butane (c4h10) that is vaporized and then burned to produce co2 a...
Questions in other subjects:

English, 22.07.2019 23:30


History, 22.07.2019 23:30








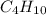 ) is a hydrocarbon used in disposable lighters. It undergoes a combustion reaction to produce a flammable gas.
) is a hydrocarbon used in disposable lighters. It undergoes a combustion reaction to produce a flammable gas.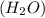 and carbondioxide (
and carbondioxide (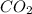 ). It is a exothermic reaction which means that heat is released during this reaction.
). It is a exothermic reaction which means that heat is released during this reaction.