
Chemistry, 08.07.2019 21:00, khadythiam6957
When solid phosphorus (p4) reacts with oxygen gas, diphosphorus pentoxide is formed. initially 2.87 mol of phosphorus and 3.86 mol of oxygen are combined. (assume 100% yield). a) write a balanced equation for the reaction. b) what is the limiting reactant? c) how many miles of excess reactant remain after the reaction is complete?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
Do you know the correct answer?
When solid phosphorus (p4) reacts with oxygen gas, diphosphorus pentoxide is formed. initially 2.87...
Questions in other subjects:




Mathematics, 18.11.2019 23:31







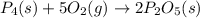
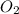 .
.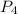 is 0.94 moles.
is 0.94 moles.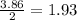 moles of
moles of 



