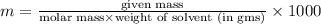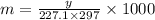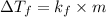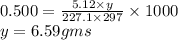Chemistry, 08.07.2019 22:00, adrianty8496
The freezing point of benzene c6h6 is 5.50°c at 1 atmosphere. a nonvolatile, nonelectrolyte that dissolves in benzene is tnt (trinitrotoluene). how many grams of tnt, c7h5n3o6 (227.1 g/mol), must be dissolved in 297.0 grams of benzene to reduce the freezing point by 0.500°c ?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 23.06.2019 05:00, rosezgomez97
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 06:00, wirchakethan23
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 10:30, dreamxette3119
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
Do you know the correct answer?
The freezing point of benzene c6h6 is 5.50°c at 1 atmosphere. a nonvolatile, nonelectrolyte that dis...
Questions in other subjects:

Social Studies, 12.10.2019 18:30



Mathematics, 12.10.2019 18:30


Mathematics, 12.10.2019 18:30

History, 12.10.2019 18:30

Biology, 12.10.2019 18:30



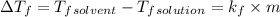
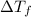 = 0.500°C
= 0.500°C 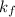 = for benzene is 5.12
= for benzene is 5.12