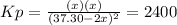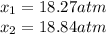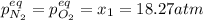Chemistry, 08.07.2019 23:30, juansebas35
for the equilibrium: 2 no (g) < > n2(g) + o2 (g), kp=2400. if initially, only no is present at a partial pressure of 37.30 atm, what will the partial pressures of n2 and o2 be at equilibrium? 1827 atm 38.08 atm 1.725 atm 36.55 atm

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
for the equilibrium: 2 no (g) < > n2(g) + o2 (g), kp=2400. if initially, only no is pres...
Questions in other subjects:

Mathematics, 01.02.2021 21:50


English, 01.02.2021 21:50






Law, 01.02.2021 21:50


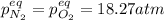
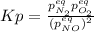
 , changes to:
, changes to: