Chemistry, 09.07.2019 00:00, cinthyafleitas
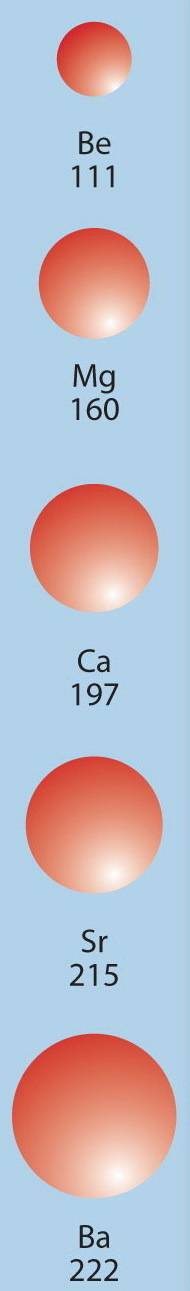
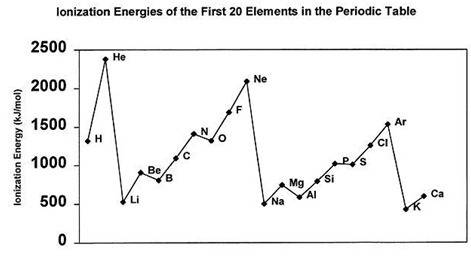
1)the metals of group 2 from top to bottom are: be, mg, ca, sr, ba. which if these metal will form ions most readily and why? 2)name the element which has the highest and lowest ionization and tell why. 3)which elements in period 2 have higher ionization energies than their immediate successor?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1


Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
Do you know the correct answer?
1)the metals of group 2 from top to bottom are: be, mg, ca, sr, ba. which if these metal will form...
Questions in other subjects:

Biology, 22.04.2021 21:20




Mathematics, 22.04.2021 21:20




Mathematics, 22.04.2021 21:20

Mathematics, 22.04.2021 21:20