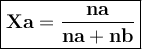Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1
Do you know the correct answer?
Asolution contains naphthalene (c10h8) dissolved in hexane (c6h14) at a concentration of 10.00 % nap...
Questions in other subjects:

Mathematics, 07.11.2019 03:31



Mathematics, 07.11.2019 03:31


Mathematics, 07.11.2019 03:31


History, 07.11.2019 03:31

Mathematics, 07.11.2019 03:31