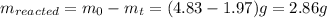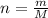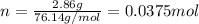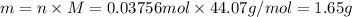Chemistry, 09.07.2019 04:30, romeojose2005
The decomposition reaction of carbon disulfide to carbon monosulfide and sulfur is first order with k = 2.80 ✕ ✕ 10−7 sec-1 at 1000°c. cs2(g) → cs(g) + s(g) a. how much of a 4.83-gram sample of carbon disulfide would remain after 37.0 days? 1.97 1.97 grams carbon disulfide b. how much carbon monosulfide would be formed after 37.0 days? 1.14 1.65 grams carbon monosulfide useful information 1.013 bar = 760 torr = 1 atm = 760 mm hg

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 23.06.2019 00:10, graceception
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
Do you know the correct answer?
The decomposition reaction of carbon disulfide to carbon monosulfide and sulfur is first order with...
Questions in other subjects:




Social Studies, 02.11.2020 17:20

Mathematics, 02.11.2020 17:20

Chemistry, 02.11.2020 17:20


Physics, 02.11.2020 17:20

Mathematics, 02.11.2020 17:20

History, 02.11.2020 17:20


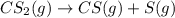
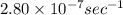 .
.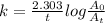
![[A_{0}]](/tpl/images/0068/1392/747e3.png) is initial concentration of reactant and
is initial concentration of reactant and ![[A_{t}]](/tpl/images/0068/1392/b9281.png) is concentration at time t.
is concentration at time t.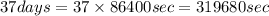
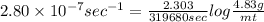
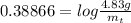
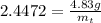
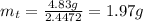
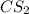 gives 1 mol of CS.
gives 1 mol of CS.