Chemistry, 09.07.2019 10:30, isiahamccoy8822
What mass of co is needed to react completely with 55.0 g of fe2o3 in the reaction: fe2o3(s) + co(g) → fe(s) + co2(g)? 4.82 g co 9.64 g co 14.5 g co 28.9 g co

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1
Do you know the correct answer?
What mass of co is needed to react completely with 55.0 g of fe2o3 in the reaction: fe2o3(s) + co(g...
Questions in other subjects:




Mathematics, 25.10.2019 04:43

English, 25.10.2019 04:43



History, 25.10.2019 04:43



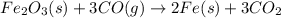
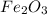 is 159.69 gram per mol and the molar mass of CO is 28 gram per mol. The calculations are shown below:
is 159.69 gram per mol and the molar mass of CO is 28 gram per mol. The calculations are shown below: