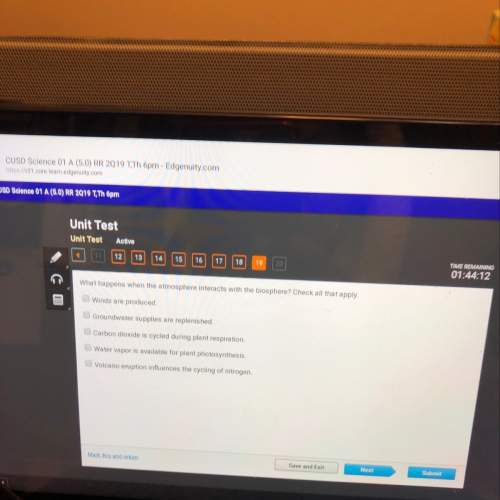
What applies to the collision theory? -particles need to collide in order to react. -particles may obtain successful collisions in any molecular orientation. -temperature increase causes kinetic energy of particles to decrease. -reactions do not have to begin with collision of molecules or particles.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, PSBSolarYT
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1



Chemistry, 21.06.2019 22:30, 20alondra04
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1
Do you know the correct answer?
What applies to the collision theory? -particles need to collide in order to react. -particles may...
Questions in other subjects:

Mathematics, 25.09.2019 16:30

Engineering, 25.09.2019 16:30


Mathematics, 25.09.2019 16:30

Chemistry, 25.09.2019 16:30




English, 25.09.2019 16:30