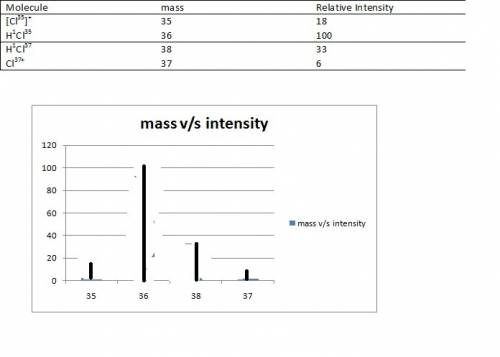
(7 pts) hydrogen and chlorine atoms react to form simple diatomic molecules in a 1: 1 ratio that is hcl. the natural abundances of the chlorine isotopes are 75.77% 35cl and 24.23% 37cl. the natural abundance of 1h is 99.985%, the natural abundance of 2h is 0.015% and there is only a trace (less than 0.001%) of 3h. (a) how many different peaks can hcl produce in a mass spectrometer and what is the mass number for each peak observed? (b) what is the relative abundance of each mass. (c) sketch the mass spectrometry analysis output (relative number of atoms vs mass number) for an hcl sample.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
Do you know the correct answer?
(7 pts) hydrogen and chlorine atoms react to form simple diatomic molecules in a 1: 1 ratio that is...
Questions in other subjects:



Mathematics, 18.03.2021 02:40

Biology, 18.03.2021 02:40

Social Studies, 18.03.2021 02:40



Mathematics, 18.03.2021 02:40