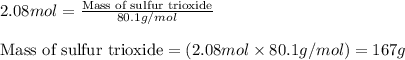Chemistry, 09.07.2019 12:30, doggosbepis
The reaction of sulfur with oxygen is shown below. 2s(s) + 3o2(g) → 2so3(g) calculate the mass of sulfur trioxide (in g) produced when 100.0 g of each reactant is present.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 22:30, safiyabrowne7594
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1


Chemistry, 23.06.2019 06:10, ridzrana02
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1
Do you know the correct answer?
The reaction of sulfur with oxygen is shown below. 2s(s) + 3o2(g) → 2so3(g) calculate the mass of su...
Questions in other subjects:



Mathematics, 27.02.2020 21:56


Mathematics, 27.02.2020 21:56





Mathematics, 27.02.2020 21:57


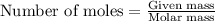 .....(1)
.....(1)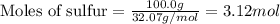
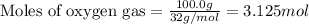
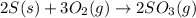
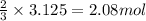 of sulfur metal
of sulfur metal