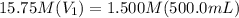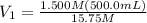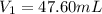Chemistry, 09.07.2019 13:30, genyjoannerubiera
Achemist needs 500.0 ml of a 1.500 m perchloric acid solution. the stockroom provides the chemist with 10.00 l of 15.75 m perchloric acid solution to prepare the required solution. calculate the volume of concentrated acid required.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3


Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
Do you know the correct answer?
Achemist needs 500.0 ml of a 1.500 m perchloric acid solution. the stockroom provides the chemist wi...
Questions in other subjects:

Mathematics, 04.11.2020 18:30


Mathematics, 04.11.2020 18:30



Mathematics, 04.11.2020 18:30


History, 04.11.2020 18:30



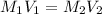
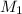 is the concentration of the concentrated solution and
is the concentration of the concentrated solution and 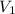 is it's volume.
is it's volume. 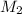 is the concentration of the diluted solution and
is the concentration of the diluted solution and 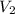 is it's volume. Let's plug in the values in the equation and solve it for
is it's volume. Let's plug in the values in the equation and solve it for