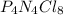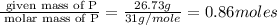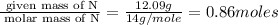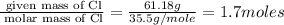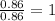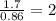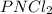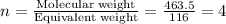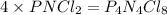“analysis of a compound of phosphorus nitrogen and chlorine show that it is 26.73% p and 12.09% n with cl accounting for the remainder. in a separate experiment, the molar mass of the compound was found to be 463.5 g/mol. determine the molecular formula of the compound.” !

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
Do you know the correct answer?
“analysis of a compound of phosphorus nitrogen and chlorine show that it is 26.73% p and 12.09% n wi...
Questions in other subjects:






Biology, 26.10.2020 18:00

Mathematics, 26.10.2020 18:00


Biology, 26.10.2020 18:00

Chemistry, 26.10.2020 18:00